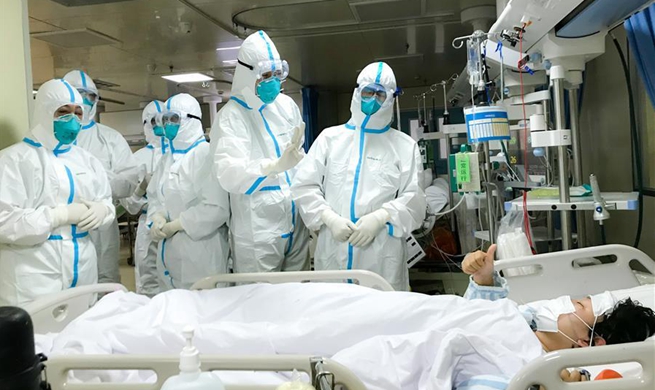
SHANGHAI, Jan. 28 (Xinhua) -- The project for the development of an mRNA vaccine targeting the novel coronavirus (2019-nCoV) has been urgently approved, said Shanghai East Hospital of Tongji University.
The vaccine will be co-developed by the hospital and Stermirna Therapeutics Co., Ltd.
Li Hangwen, CEO of Stermirna Therapeutics, said no more than 40 days will be needed to manufacture the vaccine samples based on the new generation of mRNA technology and some preliminary procedures.
The samples will be sent for tests and brought to clinics as soon as possible.
The production cycle of traditional vaccines can be as long as five to six months, whereas the mRNA vaccine has the advantage of a shorter development and production cycle.